Digital Colony Counters: Enhancing Traceability and Compliance in Microbiology
Colony counting has historically been a manual and analog process, relying on technicians to count colonies by eye—a method that, while long-established, is prone to errors and inefficiencies. With the growing complexity of microbiological testing and increasing regulatory demands, the digitalization of bacterial colony counting is emerging as a critical innovation. This transition not only improves accuracy and efficiency but also strengthens traceability and ensures compliance with federal regulations and industry standards.
Challenges in Manual Colony Counting
Manual bacterial colony counting has been a standard practice for decades, but it presents several challenges that are increasingly untenable in modern laboratory environments. Technicians must visually count colonies, a task that is time-consuming and susceptible to human error. Issues such as overlapping colonies, variations in colony size, and subjective interpretation can lead to inconsistent results. Moreover, the manual recording of data increases the likelihood of transcription errors, potentially compromising the integrity of the results.
In regulated industries—such as pharmaceuticals, food safety, and environmental monitoring—accurate bacterial counts are critical for ensuring product safety and efficacy. Errors in this process can lead to significant consequences, including product recalls, regulatory penalties, and risks to public health.
Manual colony counting with a pen
We’re here to provide you with more details.
Reach out today!
The Role of Digitalization in Colony Counting
Digitalization in bacterial colony counting addresses the shortcomings of manual methods by introducing automation, precision, and consistency. Modern automated colony counters utilize high-resolution imaging and advanced software algorithms to detect and enumerate colonies with high accuracy. These digital colony counters can distinguish between closely spaced or overlapping colonies, assess morphological features, and even categorize colonies by species based on color or size.

Automated colony counter
Digital colony counters drastically reduce the time required for counting, enabling laboratories to handle larger volumes of samples efficiently. The precision and consistency provided by digital systems help ensure that results are reliable, repeatable, and less subject to the variability inherent in manual counting.
The BC-1000 Series Automated Colony Counter can accurately and consistently count colonies as small as 50 microns on dishes, plates, and growth films! Contact us to learn more.
Contact us to learn more about how our advanced technology can help take your business to the next level.
Contact Us
Enhancing Traceability and Data Integrity in Colony Counting
Traceability is key to quality assurance in microbiology. This is especially true in regulated environments. In these settings, every step in the testing process must be documented and verified. Digital colony counters automatically generate detailed records for each sample, including high-resolution images, growth conditions, and colony counts. This data is stored electronically, creating a comprehensive and searchable database that can be easily accessed for audits, reviews, and quality control checks.
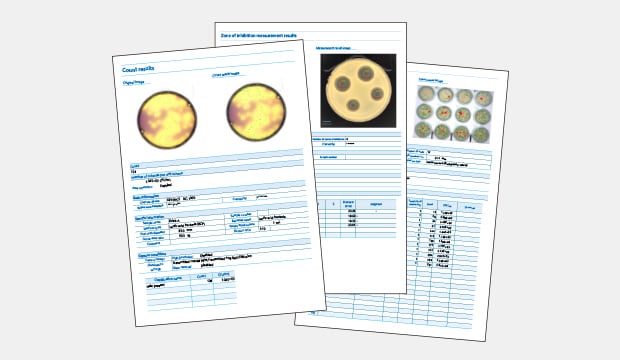
Example reports including data and sample images
By automating data capture and storage, digital colony counters reduce the risk of losing or altering data, ensuring the integrity of the results. This level of traceability is particularly important for meeting regulatory requirements, as it allows laboratories to demonstrate compliance with the applicable standards and quickly address any discrepancies that may arise.
The BC-1000 Series Automated Colony Counter captures an image of the sample being tested and archives all test results for easy access and review. The data can even be imported directly into your LIMS!
Curious about our pricing?
Click here to find out more.
Compliance with Federal Regulations and Industry Standards
Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the Environmental Protection Agency (EPA) impose stringent requirements on microbial testing practices. These regulations ensure that products, whether pharmaceuticals, medical devices, or food, are safe for public use. Compliance with these regulations often requires meticulous documentation, accurate data recording, and the ability to trace the entire testing process from sample collection to final reporting.
For instance, the FDA’s Current Good Manufacturing Practice (CGMP) regulations (21 CFR Part 211) mandate strict controls on laboratory testing procedures, including the accuracy and reliability of microbiological assays. In the context of pharmaceutical manufacturing, digital colony counters can help ensure compliance with these regulations by providing accurate and consistent results, reducing the risk of human error, and maintaining detailed records that can be easily audited.
Another related regulation, 21 CFR Part 11, is about making sure that electronic records and signatures are secure, accurate, and treated as seriously as paper records, particularly in industries like pharmaceuticals and biotechnology where safety and quality are critical. FDA 21 CFR Part 11 establishes a framework for ensuring the reliability and integrity of electronic records and signatures in these industries, supporting the move towards digital solutions while maintaining regulatory oversight and safety. Therefore, it is essential to document and maintain digital records of testing procedures, audit trails, and results.
Similarly, the EPA’s regulations on microbiological methods for environmental testing (such as those outlined in 40 CFR Part 136) require precise measurement and documentation of microbial contamination levels. Digitalization enhances the ability of laboratories to meet these requirements by improving the accuracy and reliability of bacterial counts and ensuring that all data is recorded and stored in compliance with federal guidelines.
Digital colony counters can also integrate seamlessly with Laboratory Information Management Systems (LIMS), further enhancing compliance by streamlining data reporting, ensuring that all records are easily retrievable, and facilitating the submission of data to regulatory bodies.
The standards and regulations mentioned above are just a few of the numerous requirements that industries increasingly need to meet. Other guidelines and regulations to follow include FDA BAM (Bacteriological Analytical Manual), ISO 7218, ISO 4833-2, and ASTM F2944-12 to name a few.
The BC-1000 Series Automated Colony Counter provides a robust framework for adherence to regulations and meets industry standards for microbiology testing procedures.
|
21 CFR Part 11
|
FDA BAM
|
AOAC 977.27
|
GB 4789.2
|
|
GB 7918.2
|
ISO 7218
|
ISO 4833.2
|
ISO 15189
|
We’re here to provide you with more details.
Reach out today!

The Future of Digital Colony Counting
As digital technologies continue to advance, their application in bacterial colony counting will likely expand, incorporating artificial intelligence and machine learning to further refine colony identification and differentiation. These innovations will not only improve the accuracy and speed of colony counting but also enhance laboratories' ability to comply with evolving regulatory standards.
The digitalization of bacterial colony counting is an essential evolution in microbiology, driven by the need for greater accuracy, efficiency, traceability, and regulatory compliance. By adopting digital colony counters, laboratories can ensure they meet the rigorous standards set by federal regulations, maintain the integrity of their results, and continue to protect public health and safety in an increasingly complex world.
While there are many tools available to count colonies either manually or in an automated fashion, the BC-1000 Series Automated Colony Counter uses the latest hardware and software algorithms to streamline testing procedures and provide accurate, traceable results. Even if you’ve seen an automated counter before, you’ve never seen a system like this. Contact us to experience the equipment firsthand and see how it can improve your laboratory compliance and testing workflows.
Discover more about this product.
Click here to book your demo.