Fluorescence Microscopes
Measurement of the Transfection Efficiency of Cultivated Cells
What is transfection?
Transfection is the process of introducing nucleic acids into animal cells to make the cells introduce specific genes to express a protein of interest. Nucleic acids introduced by transfection are referred to as foreign nucleic acids. Genes can be introduced to target cells without a virus, unlike with transduction, which requires a virus to transfer DNA.
Nucleic acids can be temporarily introduced to express a protein, or replicated along with the genome of the host cell. Temporary introduction of nucleic acids is referred to as transient transfection, and transfection with simultaneous nucleic acid replication is referred to as stable transfection. As such, transfection can be used to enhance or inhibit specific gene expression in the transfected cells, or to generate recombinant proteins.
Get detailed information on our products by downloading our catalog.
View Catalog
Transfection principles
The cell membrane, composed of a lipid bilayer incorporating proteins, has a negative charge that prevents large, negatively charged nucleic acids, such as DNA and RNA, from passing through. To make transfection possible, the nucleic acids must pass through the cell membrane in some other way. Other methods of allowing nucleic acids to pass through the cell membrane include chemical, biological, and physical methods, with different principles for each. This section explains each method using the introduction of a gene into a cell as an example.
Chemical transfection
With chemical transfection, the negative charge of the nucleic acid is made positive or neutralized. Methods include lipofection and calcium phosphate co-precipitation, with lipofection being the most common.
The lipofection method involves the construction of cationic lipid reagent complexes through electrostatic interactions between positively charged synthetic lipid reagent head groups and negatively charged nucleic acids. The cationic lipid reagent complexes interact with the negatively charged cell membrane surface for efficient introduction of nucleic acids into the cell. This allows the cell to take up the nucleic acids via endocytosis before being released into the cytoplasm. Lipofection is a simple and easy method of transfection with low toxicity and excellent operability.
This method enables efficient uptake of both nucleic acids, DNA, and RNA. The lipofection method can also be used for both transient and stable transfections.
For chemical transfection, however, the conditions must be appropriate for the cell type and culture, resulting in a lower transfection efficiency than physical and biological methods.
Biological transfection
The biological transfection method involves viruses. Virus-based transfection is highly efficient because it utilizes the infectiousness of a virus. This method is used for the overexpression of proteins in cells for which nucleic acid uptake is difficult. This method is ideal for in vivo transfection, making it the most commonly used method for clinical research. Adenoviruses, retroviruses, and lentiviruses are used for in vivo gene uptake.
With biological transfection, a recombinant virus is generated during gene copying, and cells transfected with genes with a helper function are taken up and propagated before extraction of just the viral vector. The viral vector containing the genes to be incorporated is then generated to deliver the desired genetic material to the cell.
Although virus-based transfection is highly adaptable for living organisms, such as for use in animal experiments, preparing the viruses can be difficult and requires adherence to biosafety standards. Insertion mutations and inactivation by the immune system are also problematic.
Physical transfection
Physical transfection involves stimulating the surface of the cell to introduce the nucleic acid through the cell membrane. Methods include electroporation, microinjection, and laser processing, with electroporation being the most common. During electroporation, the nucleic acids and the cells are suspended in a conductive solution and then clamped between an anode and a cathode. Electric pulses are then applied continuously to create small holes in the cell membrane. These holes are then used to transfer extracellular nucleic acids with an electrical charge—such as DNA and RNA—through the cell membrane.
Physical transfection offers a high transfection efficiency and is easier than chemical and biological transfection methods. However, the electric pulses may result in cell death, the holes made by the electric pulses may not close, leading to cytotoxicity, and expensive specialized equipment is necessary.
We’re here to provide you with more details.
Reach out today!

Improving transfection efficiency
Transfection efficiency is the efficiency of nucleic acid uptake by the target cells. This section introduces various factors to consider for improving the efficiency of transfection.
Cell viability and passaging
Cell viability before transfection should be at least 90%. Passaging—where the cells are transferred from the culture to the new medium—should also be performed within 24 hours of transfection. Also, note that excessive passaging can have an adverse effect on transfection efficiency, so the passage number should be carefully noted.
Cell cultures and reagents
The confluency, which indicates the ideal proliferation status of a cell for transfection, varies depending on the testing purpose, method, cell type, etc. If the confluence is too high, nucleic acid uptake may be insufficient, or transgene expression may be reduced. If the confluence is too low, intercellular contact may not occur, preventing proliferation.
The optimal medium for growing cells for transfection depends on the cells, reagents, and cell type serum. When introducing DNA into stem cells, for example, reagents capable of introducing nucleic acids into embryonic stem cells (ES cells), induced pluripotent stem cells (iPS cells), human adult stem cells, and other types of cells with high efficiency and low toxicity are used. When introducing RNA into stem cells, reagents capable of introducing siRNA (small interfering RNA), mRNA (messenger RNA), and dsRNA (double-stranded RNA) while protecting the stem cells from degradation are used.
Transfection method selection
Transfection methods include chemical, biological, and physical transfection. Chemical and physical transfection are commonly used for targeted transfection of cultured cells. Biological transfection is effective for transfection of cultured cells and animal cells. However, chemical transfection can cause variations in transfection efficiency or chemical toxicity depending on the cell type and conditions, and selective transfection can be difficult with some cells. Meanwhile, physical transfection requires special instruments and equipment, and nucleic acids are easily damaged. Biological transfection also carries a risk of contamination, insertion mutations when introducing therapeutic genes into the cell chromosomes, and immunity-based inactivation.
Because each method has disadvantages, selecting a transfection method with a high transfection efficiency and high reproducibility for the specific combination of transfection cells and target cells is essential.
Other factors
Transfection efficiency also varies depending on whether serums or antibiotics are used. For example, when introducing DNA, culturing the cells in a medium with serum increases efficiency. Performing transient transfection in a medium with antibiotics also increases transfection efficiency.
However, antibiotics can also cause cytotoxicity that can adversely affect the cells and reduce transfection efficiency. Moreover, DNA and RNA vectors can both be used for transient transfection, but only DNA vectors can be used for stable transfection.
We’re here to provide you with more details.
Reach out today!

Measuring transfection efficiency
One general method for measuring transfection efficiency is to use a fluorescence microscope. The transfection efficiency is measured by counting the total number of observed cells and the number of cells that express fluorescence, and scoring these values.
Transfection efficiency measurement
During gene introduction, seed a cell suspension prepared with a growth medium for proper cell density into a well plate and incubate. Then, add the prepared complex to the cells, shake the well plate, and incubate the cells again for 24 hours in an incubator. When incubation ends, observe the expression of the cells to measure transfection efficiency. Transfection efficiency is commonly measured using a fluorescent dye and a fluorescence microscope.
Transfection efficiency measurement challenges
Conventional fluorescence microscopes pose various problems and are often difficult to use.
First, observing the fluorescence requires taking the specimen to a darkroom and performing the experiment there, resulting in extremely poor operability. Accurately extracting the outlines of cells is also difficult, hindering the accurate counting of the number of cells.
In addition, to count and analyze the number of cells, software separate from the fluorescence microscope system is required, leading to many customers noting the difficulty in performing smooth analysis.
The BZ-X All-in-One Fluorescence Microscope includes a built-in specimen enclosure that allows users to perform fluorescence imaging in a brightly lit room. The BZ-X accurately extracts and quantifies phase-contrast image contours, even for cultured cells with subtle luminance differences, and allows users to specify a cell mask area for measuring the number of measurement targets and the surface area ratio. The BZ-X can also measure cell expression levels for quantitative measurement of transfection efficiency without needing separate software.
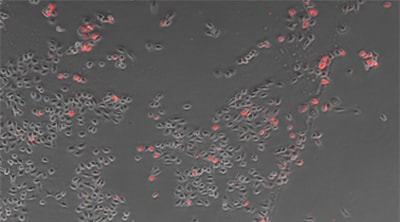
Counting cells using a phase contrast image
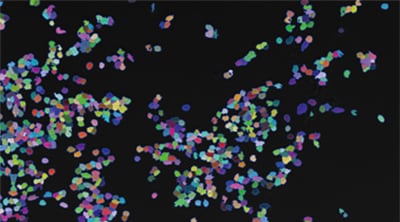
Counting expression events (fluorescent points) using cells as mask areas
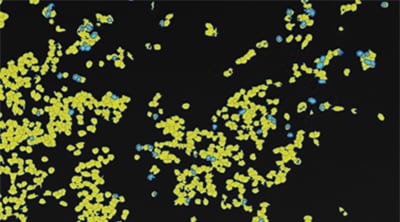
Cell:903 Expression:89 Efficiency:9.9%
Objective lens: CFI Plan Fluor DL 10x
Using the All-in-One Fluorescence Microscope BZ-X
- Since no darkroom is required, easy fluorescence imaging can be done in any laboratory setting. A low-photobleach imaging mode can be used to minimize damage to the cells.
- Both phase contrast and fluorescence images can be captured and then overlaid for a more comprehensive view.
- Whether using a phase contrast or fluorescence image, our proprietary measurement software can quickly extract the outline of the cell and accurately count or measure their characteristics.
- Hybrid Cell Count can be used to extract the fluorescent protein contained in the cells and count them by using cells as mask areas.