Continuous Inkjet Printers / Case Coder
Materials and Products
Production Machines
Printing Applications
Benefits of Pharmaceutical Date Coding in Improving Traceability
The US Food and Drug Administration (FDA) and Drug Supply Chain Security Act (DSCSA), as well as EMA and FMD (European equivalents), mandate comprehensive traceability measures for all perishable products, including pharmaceuticals.
These regulations require detailed tracking of medical products, including both equipment and pharmaceutical products, through serialized and scannable codes to enhance supply chain security and ensure consumer safety.
Traceability is the ability to track each step of the pharmaceutical product journey through the supply chain, from the manufacturer to the distributor, retailer, and the end consumer. It's a vital system that ensures that medication is safe, effective, and free from any contamination or tampering, ultimately protecting the safety and health of end consumers.
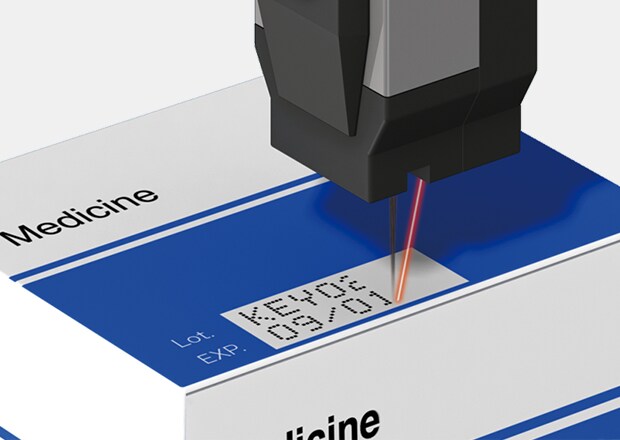
Thus, pharmaceutical date coding involves marking packages with the product’s manufacturing date, expiration date, and batch number. Since clear, accurate, and easily decipherable lot and date coding is crucial for maintaining the integrity of the product, the pharmaceutical date coding practice is subject to some of the strictest standards globally like GS1.

Discover more about this product.
Click here to book your demo.
Ensuring Product Authenticity
Counterfeit pharmaceuticals, or, simply put, fake drugs, pose a significant threat to public health, as they often contain incorrect ingredients or dosages. Pharmaceutical serialization and traceability are the first line of defense in identifying counterfeit pharmaceuticals and preventing dangerous substances from reaching consumers.
When first receiving pharmaceutical products through the supply chain, retailers and pharmacies scan the products for pharmaceutical packaging date coding, which contains important data about the product. These scans are then cross-referenced with the upstream participants in the supply chain, who go back to the source of said products, whether they're the manufacturer or the wholesaler.
Date coding ensures that the products are authentic, making it much harder for counterfeit pharmaceuticals to infiltrate the market. This is essential, as it ensures that consumers receive safe pharmaceuticals that meet regulatory standards.
We’re here to provide you with more details.
Reach out today!

Quality Control and Safety
Besides ensuring product authenticity, pharmaceutical coding plays an important role in quality control as the products move through production and down the supply chain. Date codes are implemented so that products and entire batches can be more easily identified and inspected for compliance with safety standards.
Adequate date coding on the product ensures quick detection of expired or contaminated products, which prevents their sale and consumption and acts as a proactive measure that significantly enhances product safety.
It also helps verify that the product has been stored and handled correctly throughout the supply chain, maintaining its efficiency and safety of use. If any issues or discrepancies in production or logistics are detected, these codes ensure the products can be traced throughout the supply chain, which allows for a fast and efficient recall process, maintaining public safety and brand reputation.
Curious about our pricing?
Click here to find out more.
Minimizing Downtime and Errors
Pharmaceutical serialization and traceability are applied by the manufacturer's packaging department or a company that handles packaging. The process is highly automated, with printers such as KEYENCE's MK-G Series CIJ printing serialized date and lot numbers at exceptionally high speeds.
But the use of automation transcends utilitarian reasons, and it's not only about speed but also precision. Namely, printers such as the MK-G Series ensure the application of reliable, high-quality, and accurate pharmaceutical date coding with machine precision, lowering the risk of errors in the coding process.
This ensures consistency and accuracy of pharmaceutical serialization and traceability, which is essential for regulatory compliance and consumer safety. Automated date coding systems also optimize the production process and reduce the waste associated with printing and coding errors.
They also significantly reduce downtime, which further maintains the efficiency and safety of the entire manufacturing process and eliminates the need for manual coding and associated errors.
![Expiration date mistake categories: [1] 81%, [2] 16%, [3] 3%](/Images/benefits-of-pharmaceutical-date-coding-in-improving-traceability_img_03_2148847.jpg)
Among all the errors caused by expiration date mistakes, 81% were caused by the date being displayed incorrectly. In another 16% of the cases, printed text was missing in whole or in part.
-
1Incorrect date printed
-
2Missing printed text
-
3Other
Get detailed information on our products by downloading our catalog.
View Catalog
Customer Confidence and Brand Reputation
As previously stated, pharmaceutical date coding conveys plenty of different information to all the links within the supply chain; one of those details is the expiration date, which denotes that the product is effective and safe to use, playing a direct role in consumer safety and satisfaction.
Adequate coding also helps those running the supply chain to minimize the frequency and scope of product recalls by ensuring that all products are adequately managed, which ensures customer satisfaction and loyalty.
For the customers, however, pharmaceutical date coding ensures that the quality and safety of the product they bought is valid, enhancing their trust in the brand and the manufacturer.
Discover more about this product.
Click here to book your demo.

Enhanced Traceability with KEYENCE's MK-G Series for Pharmaceutical Packaging Date Coding
CIJ printers play a crucial role in date coding for various industries, including those dealing with perishable goods, such as the pharmaceutical industry, with its strict regulatory demands and fast-paced production.
Fortunately, the KEYENCE MK-G Series is known for its precision, reliability, and quality of print in pharmaceutical coding, thanks to a series of fantastic features that continuously monitor break points to ensure perfect dot quality for printed content.
It is designed for seamless integration into existing manufacturing settings. Its ease of use and built-in diagnostics make it accessible for anyone to operate, eliminating the need to rely solely on skilled or experienced maintenance technicians. This allows skilled maintenance employees to focus on more critical roles.
The MK-G Series also has a self-troubleshooting option, in which the printer performs self-diagnostics, cleans itself, and/or notifies the staff. This is particularly important, as it helps eliminate inconsistencies in print quality, ensures compliance, and reduces the costs associated with unplanned maintenance and downtime.
It's also noted for its high-speed throughput/marking and compatibility with various packaging materials, from glass and metal to a range of plastics such as PET, PP, PE, and many others.
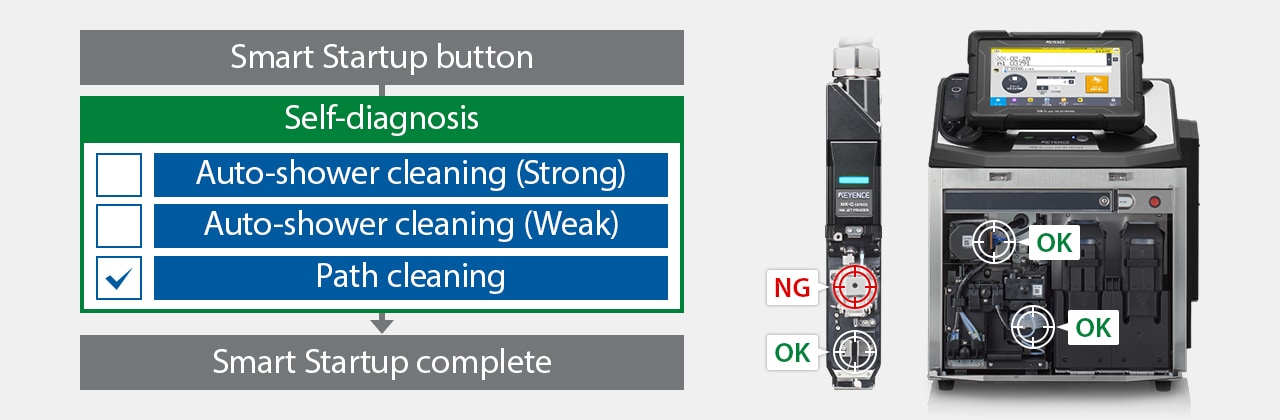
Using the MK Dock enables self-diagnosis and cleaning to ensure stable startup.
Get detailed information on our products by downloading our catalog.
View Catalog

Why KEYENCE?
KEYENCE is the world's leading provider of cutting-edge precision measurement sensors and technologies that are widely used in the pharmaceutical industry. So, if you're looking to enhance your packaging and traceability systems, the KEYENCE MK-G Series offers a robust solution to support compliance, ensure product safety, and maintain high operational efficiency.
Don't hesitate to contact KEYENCE and inquire about possible integrations of our equipment with your current lineup.
We’re here to provide you with more details.
Reach out today!

Related Downloads

This guide explains continuous inkjet (CIJ) printer applications together with pictures and illustrations. It contains many installation examples in various industries divided into food, medicine, and cosmetics; electrical machinery and electronics; and automotive, metal, and others. These examples show printing on targets specific to each industry.
Related Products
Applications
Materials and Products
- Glass Bottles
- Plastic Bottles/Containers
- Cans
- Wires, Cables, & Pipes
- Pouches
- Metal Parts
- Corrugate Boxes
- Packaging Film
- Kraft Packaging
- Folding Cartons
- Building Materials
- PCBs
- Cartons
- Pallets & Lumber
- Corrugate Trays
Production Machines
- Paper Bag Packaging
- Box Conveyor
- Automatic Palletizer
- Sealer/Taping Machine
- Bottle Filling Machine
- Flow Wrapper


![Process-specific Marking Applications [Food/Pharmaceutical Industry]](/img/asset/AS_133442_L.jpg)

