Fluorescence Microscopes
Cell Migration Assay
Aside from red blood cells, which carry oxygen to all corners of the body by traveling in blood, the majority of the vast number of cells that make up the living body serve their functions without moving from their specific locations. In contrast, there are cells that actively travel in both blood and body tissues, such as immunocytes. This page explains cell migrations and the assays used to assess them.
Get detailed information on our products by downloading our catalog.
View Catalog
Cell migration and how to assess it
Cell migration is the movement of cells from one location to another. For example, cell migration can be observed in the process of wound healing as a scratch or minor injury in the epidermis heals. When the skin sustains a wound, the clotting factor in the blood is activated to form a blood clot at the wound site to arrest the bleeding (coagulation phase). Next, as part of the immune response, inflammatory cells such as neutrophils, macrophages, and lymphocytes migrate to the wound and removes the dead tissue (inflammatory phase). The substance released by macrophages recruits the fibroblasts in the area to migrate to the wound, generating collagen for repair and promoting angiogenesis to form granulation tissue (proliferative stage). Finally, the epidermal tissue cells migrate, which makes the wound edges contract and close (remodeling phase). A wound heals through these phases.
Cells navigating through extracellular matrix (ECM) and penetrating neighboring tissues is called cell invasion. For example, when inflammation occurs due to bacterial or viral infection, as part of the body’s defensive reaction, the inflammatory cells such as neutrophils, macrophages, and lymphocytes intensively migrate toward the inflammation site and infiltrate the inflammatory tissue, causing inflammatory cell infiltration. Other types of cell invasion include tumor invasion (cancer cell invasion), where tumor cells such as cancer cells infiltrate and spread in neighboring tissue or organs.
Cell migration plays an important role in wound healing, cell differentiation, and embryonic development. On the other hand, if some kind of error occurs during cell migration, it can lead to serious results such as tumor formation, tumor metastasis, and/or cancer metastasis. As such, cell migration is involved in different processes in the body.
Types of cell migration assays
Cell migration assays allow you to examine the migration capabilities or mechanisms of invasion of research target cells, or study the factors and conditions that promote cell migration and invasion. Cell migration assays are used to study cell migration enhancements caused by pharmaceuticals and cosmetic products, in research into tumors and cancer metastases and research and development of drugs to control them, and other therapeutic methods. This section introduces the typical types of cell migration assays used to measure and assess cell migration and invasion.
Chemotaxis assay
Immune cells such as white blood cells (neutrophils), macrophages, and lymphocytes, as well as fibroblasts, endothelial cells, epithelial cells, and tumor cells, are chemotactic cells, or cells that migrate according to the concentration gradient of certain chemicals that exist around them. Their cell migration is triggered by chemokine—a type of cytokine—and other chemoattractants as the chemotactic factor. When cell migration occurs toward a higher chemical concentration, it is called positive chemotaxis.When cell migration occurs toward a lower chemical concentration, it is called a negative chemotaxis, and the chemicals that cause such reactions are called chemorepellents. In a cell migration assay that examines chemotaxis, cells that pass through the membrane insert inside the well and adhere to the bottom where the chemotactic factor is placed, are migratory cells, and cells that do not pass through the membrane insert and remain above the filter are non-migratory cells.
Haptotaxis assay
In a cell migration assay that evaluates haptotaxis, a membrane insert whose bottom (back side) is coated with collagen or fibronectin—chemoattractants that are chemotactic factors to fibroblasts, endothelial cells, and epithelial cells—is placed inside a well to examine whether cells are attracted to and migrate toward these chemicals in order to determine whether the cells are haptotactic or non-haptotactic. Additionally, the concentration gradient of the chemoattractant bound to the ECM or cell adhesion site can be used to measure the migration capability of different cells.
Wound healing assay
This is an in vitro assay that replicates the conditions of wound healing in a multi-well plate. In a typical method called the scratch assay, immune cells that migrate and infiltrate an intentionally made wound are measured for evaluation. Measurement of cell infiltration in repair of wounds, including tissue matrix production in wound healing, differences in the cells involved, and cell proliferation and infiltration in different culture conditions, can be useful in research, development, and assessment of pharmaceuticals.
Cell invasion assay
Cancer cells eventually separate from where they first formed and infiltrate neighboring tissues by destroying the basement membrane. Then the cancer cells migrate into the blood vessels to be carried away in the blood to different parts of the body. Cancer metastasis occurs when cancer cells proliferate at the location that they were carried to. When cancer cells infiltrate tissue, protein hydrolysis and cell migration occur in the cell adhesion, basement membrane, and ECM. By using this property, cell tumors can be identified and measured using a membrane insert coated with collagen, laminin, or basement membrane matrix solution. Cell infiltration can be evaluated and substances that can inhibit infiltration can be studied by measuring infiltrating cells in an in vitro environment that promotes cell infiltration using a 24 or 96-well plate. Cells are stained after culturing in order to identify and quantify cell tumors.
We’re here to provide you with more details.
Reach out today!

Issues in assessing cell migration capability and their solutions
Cell migration assays are performed by using an assay kit suitable for the purpose, to evaluate cell migration capabilities and other properties by measuring cell migration and infiltration. A 24 or 96-well plate is used to ensure the necessary number of samples under the same environmental conditions, but analyzing and measuring numerous samples with a conventional microscope and manually-operated counter can take a lot of time and effort. Simultaneous analysis and measurement of the status and changes in live cells in many wells is not only difficult, but also susceptible to human-related measurement errors as well as variations in evaluation. Even when using an automatic cell counter, the transfer of samples containing live, changing cells can be troublesome. Identifying different cells based solely on size can be difficult in some cases.
Accurately analyzing and assessing cell migration
KEYENCE’s BZ-X all-in-one fluorescence microscope can perform automatic and continuous time-lapse imaging and video capture of each well of a multi-well plate. The auto-focus and full-focus functions help you capture clear images or videos of each well.
After video capture, you can run a video analysis (motion analysis) on each of the many wells using an analysis application. By just clicking the measurement target to specify the analysis range, you can analyze cell migration capabilities such as chemotaxis and haptotaxis while recording changes in the location coordinates obtained through automatic following of the target. Time-lapse image capture and video capture allow you to see the progress of cell migration and infiltration at certain intervals over time even while imaging is ongoing.
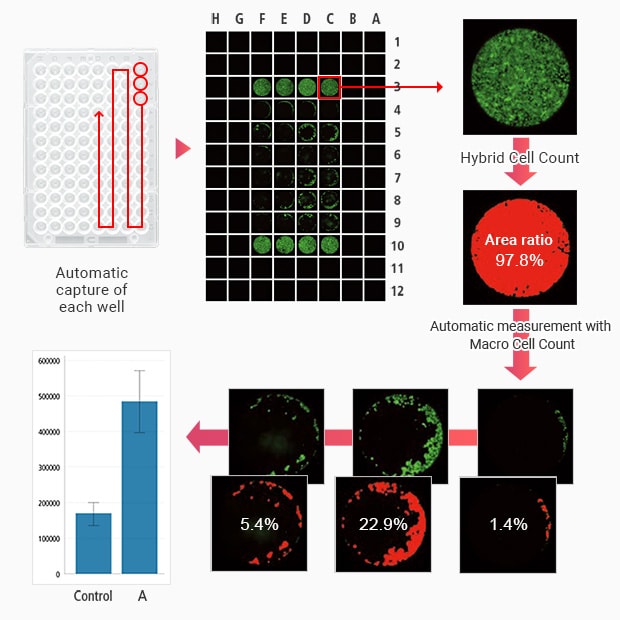
The Hybrid Cell Count function accurately measures the area of only the signal part in each well. The Macro Cell Count feature offers automatic measurement where the user only needs to measure a single image to apply the same conditions to the rest of the images, which makes quick quantitative measurements possible.
The BZ-X provides a broad range of functions that not only drastically shorten and streamline the time previously spent on analytical tasks to assess cell migration and infiltration in multi-well plates, but also help improve the reliability of analysis data thanks to its precise quantitative measurements.
Using the All-in-One Fluorescence Microscope BZ-X
- The BZ-X can perform automatic and continuous time-lapse imaging and video capture of each well.
- The auto-focus and full-focus functions help you capture a clear focused image or video of each well of a multi-well plate.
- Time-lapse image capture and video capture allows you to examine the progress of cell migration and infiltration at certain intervals over time.
- Hybrid Cell Count can be used to accurately measure the area rate of only the signal part in each well.
- With Macro Cell Count, you can apply the same measurement conditions you used for one well to the images from other wells, which allows for batch measurement of a large number of wells with the same conditions.
- Thanks to its ability to provide quantitative measurements with uniform measurement conditions, the BZ-X produces highly reliable analysis results with no arbitrariness.