Fluorescence Microscopes
Primary purpose of microbial testing
Microbial testing is broadly classified into testing for the sanitation and safety of products, such as foods and cosmetics, and medical testing related to infectious diseases.
Here, we will explain the purpose of microbial testing of foods that are directly taken into the body.
Microbial testing of foods and its purpose
Microbial testing of foods is conducted by food businesses for the purpose of food safety control, specifically to examine microbial counts to check for the presence of any bacteria or fungi that are responsible for food poisoning and to assess the sanitation status.
Microbial testing is regulated by standards and guidelines, and food businesses are mandated to ensure and verify food safety throughout the food chain—from procurement of raw ingredients all the way through manufacturing, transportation, and storage—by testing according to applicable standards.
Sanitation control and quality/safety evaluations are performed during product manufacture to prevent problems. Additionally, if a complaint arises from consumers after shipment or in the case of a food poisoning incident presumed to be caused by manufactured food, the remaining food, ingredients, cooking equipment and tools used for manufacturing, and the facilities are swabbed and sampled for testing to identify the cause of the incident and to prevent recurrence.
Get detailed information on our products by downloading our catalog.
View Catalog
Common methods for observation of microbes
Microbes that are tested for in the food and cosmetics industries are generally clear and colorless. To observe microbes for identification, detection, and/or evaluation, an observation and analysis method suitable for clear specimens must be used. This page explains typical optical and staining methods for observing and analyzing microbes.
Differential interference contrast (DIC) observation
What is differential interference contrast observation?
Differential interference contrast (DIC) observation is a method of observing unstained, transparent samples using optical path differences. For example, when light passes through a transparent microbial sample, the distance that the transmissive light travels (optical path) differs depending on the difference in the thickness and the refractive index of the specific part of the sample. This is referred to as the optical path difference. This creates contrast according to the gradient in the thickness of the sample, producing a three-dimensional image. The shadows (contrast) have directionality, which can be finely adjusted for observation.
Mechanism and working principle of DIC observation
A DIC microscope is equipped with a differential interference contrast (DIC) prism on both the condenser lens and objective lens sides. Polarizing plates are arranged on the outside of the DIC prism, on both the condenser lens and the objective lens sides, respectively called the polarizer and analyzer. The light emitted from the light source becomes polarized—light oscillating in only one direction—when it passes through the polarizer. The polarized light branches into two beams of oscillating polarized light through the condenser-side DIC prism. These beams are mostly parallel when emitted on the sample. The polarized light that passed through the thick part of the sample and the polarized light that passed through other parts have an optical path difference. The two beams of polarized light that have passed through the sample meet at the DIC prism on the objective lens side and pass through the analyzer. A DIC microscope allows you to observe the interference arising from the optical path difference of the two polarized beams as light-dark contrast.
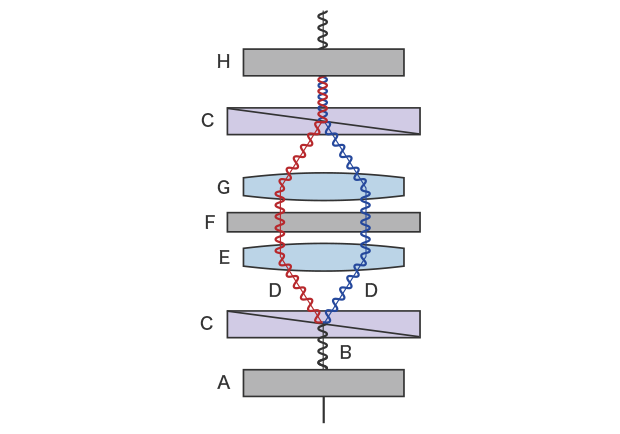
A: Polarizing plate (polarizer) B: Illumination light C: DIC prism D: Polarized light split into two beams E: Condenser lens F: Sample G: Objective lens H: Polarizing plate (analyzer)
Schematic diagram of differential interference contrast observation
Precautions for DIC observation
When light strikes a plastic container, the light polarizes in the container; therefore, you must use a glass container for DIC observation. Contrast is also generated by any foreign matter on the sample or lens, resulting in noise, so any contamination must be removed before observation. Shadows appear (contrast) in the observation image in a certain direction, which means that it is important to turn the stage along with the sample to change the direction of the contrast in some cases.
Phase contrast observation
What is phase contrast observation?
Phase contrast observation is an observation method that uses the shift in the phases between diffracted light* and direct light that occurs when light from the light source passes through the sample. The conversion of this phase contrast (optical path difference) into light-dark contrast allows for observation of transparent samples such as unstained microorganisms.
Mechanism and working principle of phase contrast observation
Microscopes that offer phase contrast observation have a phase plate and a ring slit (ring aperture) on the optical path. This ring slit shapes the light from the light source into a cone. The annular light passes the annular phase plate on the objective lens side through the condenser lens and sample.The phase plate generates a shift in the phase of the light that passes through the sample.
When the sample is placed on this optical path, the light passing through the sample splits into direct light and diffracted light* due to diffraction* of light. When these two beams of light with different phases pass through the phase plate from the objective lens, they enhance or weaken each other due to wavelength interference. In phase contrast observation, the interference from the phase difference is visualized as a contrast between the object image and background.
*Diffraction (diffracted light): As light has a wave nature, when there is an obstacle (sample) in the optical path, part of the light does not travel straight, but bypasses the obstacle. This is called diffracted light.
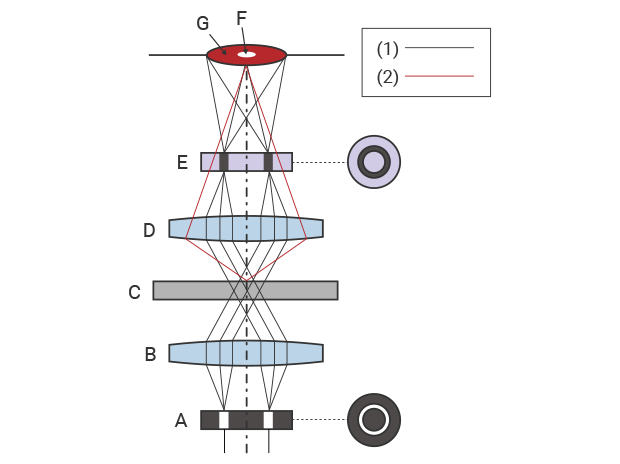
1: Straight light 2: Diffracted light A: Ring slit (ring aperture) B: Condenser lens C: Sample D: Objective lens E: Phase plate F: Object image G: Background
Schematic diagram of phase contrast observation
Precautions for phase contrast observation
If the center of the ring slit is misaligned, the annular light leaks from the phase plate, weakening the phase shift of the direct light and thus weakening the contrast. Additionally, if the condition of the prepared slide differs between samples, center alignment of the ring slit must be performed for every sample exchange.
Gram staining
What is Gram staining?
Gram staining is a staining technique used to detect and identify clear, colorless microorganisms and bacteria. Unlike optical methods such as differential interference contrast observation and phase contrast observation, transparent samples are stained or decolored using reagents to distinguish the cell wall structure and components of bacteria, which enables identification and analysis based on their color and shape.
Example of Gram staining procedure: Microbial testing of food
Three typical Gram stain methods are: Hucker’s modified method, the Favor method (Nishioka’s method), and the Bartholomew and Mittwer’s method. The following explains the Gram stain procedure using an example of microbial testing of food using Hucker’s modified method, which is considered to be the standard method of staining.
(1) Smearing
Smear a pure culture of microorganisms onto a slide that has been degreased and loaded with sterile purified water. After air-drying, pass the slide through weak flame with the smear-side facing up (heat fixing). Wash the slide from the back side (water rinsing). Another way of adhering the smear to the slide is alcohol fixation, where the slide is flooded with methanol after drying.
(2) Staining
Apply crystal violet (primary stain) to the slide and rinse the slide. Then, apply Lugol’s iodine solution to the smear and rinse again.
(3) Decolorization
Decolorize using 95% ethanol (or acetone). Rinse both sides of the slide thoroughly so that no ethanol or acetone is left on the smear.
(4) Counter-staining and drying
Counter-stain using safranin (or carbol-fuchsin) and rinse again. Drain the water off the smear, blot dry the slide with bibulous paper, and let it air-dry completely.
(5) Microscopic test
Oil-immersion lenses are commonly used for high-magnification microscopy.
Identifying microorganisms based on stainability, shape, and morphology
The following are examples of classification and identification of microorganisms and bacteria based on stainability, shape, and morphology using Gram staining.
| Microorganisms/Bacteria | Classification/Identification | |
|---|---|---|
|
|
Gram-positive bacteria
|
Stained blue (Cell walls are thick and not prone to decolorization)
|
|
Gram-negative bacteria
|
Stained red (Cell walls are thin and prone to decolorization)
|
|
|
|
Gram-positive cocci
|
Staphylococci (bacteria that form in grape-like clusters), Streptococci (bacteria that form chains), etc. Staphylococcus aureus is a typical causative agent for food poisoning.
|
|
Gram-positive rods
|
Corynebacterium (club-shaped, palisade, V-shaped, or W-shaped arrangement), Bacillus (appear as thick squares or trapezoids), etc.
Clostridium perfringens, Bacillus cereus, and Clostridium botulinum are typical causative agents for food poisoning.
|
|
|
Gram-negative cocci
|
In many cases, causative bacteria for infectious diseases are determined from sputum specimens.
|
|
|
Gram-negative rods
|
Typical bacteria in this classification include intestinal bacteria (e.g. Escherichia coli), which are larger compared to other Gram-negative rods and are stained darker on both ends, and non-glucose-fermenting bacteria (e.g. Pseudomonas aeruginosa), which are thin and relatively small. Causative bacteria for food poisoning include Vibrio parahaemolyticus, salmonella, campylobacter, enterohemorrhagic E. coli, Shigella, and Vibrio cholerae.
|
Precautions for Gram staining
Over-decolorization or other errors in decolorization may result in improper stain-differentiation of Gram-positive/negative bacteria, which may cause incorrect assays. Additionally, a single field of view may not provide sufficient information for a correct assay. It is important to make a comprehensive assessment by observing and analyzing the sample from multiple fields of view.
We’re here to provide you with more details.
Reach out today!

Observation and quantitative analysis of microbes using a fluorescence microscope
As microorganisms are mostly clear and colorless, it is common to introduce contrast to images by means of differential interference contrast or phase contrast microscopy. Gram staining is another typical visualization method used to make assessments from microbial count, stainability, shape, and morphology. However, with these methods it takes a lot of time to evaluate the status and shapes of microbes that exist in large numbers on the screen or to visually perform a microbial count under a microscope, making quantitative analysis difficult. Another issue is the difficulty in examining how long microbes live and the temporal transition of the ratio of live microbes to dead microbes.
The solution: Microbial testing and examination using a fluorescence microscope
In general, microbial testing of food, cosmetics, and quasi-pharmaceutical products targets live bacteria. A fluorescence microscope, which is a type of biological microscope, uses fluorescent antibodies or proteins as labels that enable observation of live microbes without damaging their cells or proteins.
KEYENCE’s BZ-X All-in-One Fluorescence Microscope allows for non-staining observation of microbes, which not only drastically shortens sample preparation time, but also eliminates any damage to microbes caused by staining. The BZ-X also makes observation, quantitative analysis, and time-series measurement of Gram-stained microbes easy. In particular, the BZ-X can significantly reduce time and labor in cases where large volumes of comprehensive data are required to complete an assay or evaluation, such as bacterial count over multiple images collected over time.
For evaluation of surfactants and preservatives in cosmetic and quasi-pharmaceutical products, as well as for viability tests such as those for effective counting of lactobacillus in food, the BZ-X can shine light on cell membranes to check for live microbes and display dead microbes as red by means of changes in their brightness value. The changes in brightness value are expressed and recorded as live-cell imaging (time-lapse imaging) and can be used for real-time observation and measurement at any point in time.
The BZ-X’s features not only facilitate quantitative analysis and evaluation of food, cosmetics, and quasi-pharmaceutical products through microbial testing, but also allow for recording of easy-to-understand videos that show the functioning of lactobacillus and yeast in foods, which can be applied as promotional tools that appeal to consumers.
In addition, the BZ-X can use the darkroom inside its enclosure for observation and analysis, so you don’t have to prepare a separate darkroom. This also offers flexibility in installation location when introducing the instrument for in-house testing.
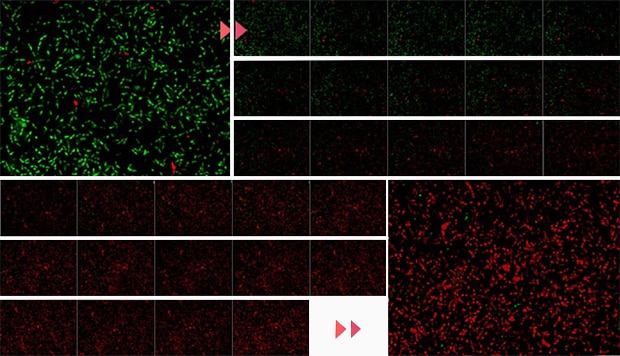
Changes in brightness against the screen
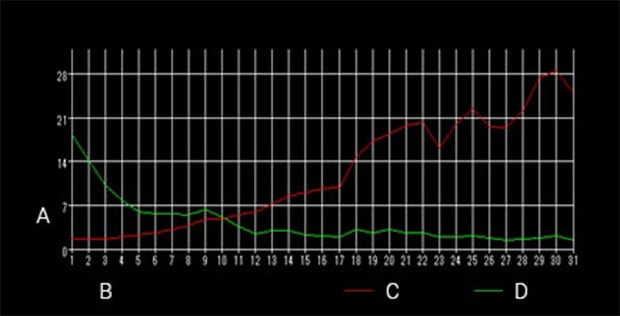
A: Brightness B: Time (number of images) C: Red brightness D: Green brightness
Time-series measurement
Green: Live bacteria
Red: Dead bacteria
Imaging interval: 20 seconds
Imaging time: 10 minutes
Using the All-in-One Fluorescence Microscope BZ-X
- KEYENCE’s BZ-X All-in-One Fluorescence Microscope allows for observation and quantitative analysis of microbes without staining them, which not only saves on sample preparation and analysis time, but also eliminates damage to samples caused by staining.
- The BZ-X can simultaneously measure the microbial count from multiple time-series images of a Gram-stained sample for analysis and evaluation.
- Based on changes in the brightness value of cell membranes, the BZ-X can present a color-coded display of live and dead microbes, which allows it to provide the temporal transition in microbial viability ratio as numeric data, graphs, and videos.
- Thanks to its ability to express the changes in the ratio of live to dead microbes in an easy-to-understand video, it can aid in creating product promotions targeting consumers.
- A darkroom is not necessary, so it allows for a range of installation possibilities for in-house testing.