Fluorescence Microscopes
What is a micronucleus test?
This page explains genotoxicity tests (mutagenicity tests), a type of safety testing for food and pharmaceuticals, and provides an outline and the principles of micronucleus testing, a typical genotoxicity test.
What is genotoxicity and what are genotoxicity tests?
First, let’s explore what genotoxicity is and the tests that examine its presence.
Genotoxicity is the property or effect of chemical agents or radiation that interact with the chromosomes or DNA proteins in cells, causing mutations in these structures. Even if a chemical agent exhibited genotoxicity, that chemical on its own does not cause the effect. When a genotoxic agent interacts with somatic cells, it has potential toxicity that may lead to cancer or other diseases, or in some cases, trigger hereditary diseases that are passed on across generations. A property that induces irreversible, heritable change (mutation) to the DNA is called mutagenicity. On the other hand, genotoxicity is used as a broad term that also includes the property that induces changes in DNA and chromosomes that are not heritable.
Genotoxicity tests are conducted to evaluate whether a chemical agent used in a pharmaceutical, additive, agricultural chemical, or general chemical product potentially exhibits genotoxicity. In general, a test substance is administered to a mammal or the cultured cells of a mammal, and any DNA damage or chromosomal aberrations from the interaction are examined for toxicity assessment. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) establishes guidelines for pharmaceutical regulations, among which are international guidelines for genotoxicity testing for pharmaceuticals. Normally, genotoxicity tests are conducted and the test results are assessed in accordance with the ICH guidelines. These guidelines are discussed and revised by expert working groups while considering the opinions of regulatory authorities of the countries and territories that are part of the ICH.
What is a micronucleus test?
A micronucleus test is a genotoxicity test that evaluates the presence of micronuclei in cells that have been administered a test substance (chemical, etc.) and the frequency of cells harboring micronuclei among normal cells. It is used as a genetic toxicology test to identify chromosomal aberrations. A micronucleus is a small nucleus that forms whenever a fragment of a chromosome is not incorporated into one of the daughter nuclei during cell division as result of a chromosomal aberration induced by an interaction with a chemical.
Micronucleus tests are classified into two groups based on the target to which the test substance is administered:
- In vivo micronucleus assays: micronucleus assays that use mammals such as hamsters, mice, and other rodents.
- In vitro micronucleus assays: micronucleus assays that use cultured mammalian cells such as CHL/IU cells (Chinese hamster lung fibroblast cells).
The principle of micronucleus formation in micronucleus testing
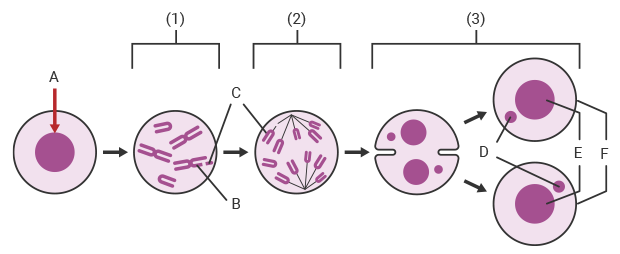
A:Administration of test substance B:Chromosome breakage C:Fragment D:Micronucleus E:Daughter nucleus F:Daughter cell
The following figure shows the principle of how micronuclei form in cells during a micronucleus test when a test substance exhibits genotoxicity.
-
1Metaphase: If the test substance administered to a mammal or the cultured cells of a mammal is mutagenic, a chromosomal aberration such as chromosome breakage occurs.
-
2Anaphase: Cell division progresses with the chromosome fragment remaining inside the cell.
-
3Telophase: Chromosome fragments that were not incorporated in the daughter nuclei remain separately as micronuclei inside the daughter cells.
* Some micronucleus tests use cytochalasin B to prevent division into daughter cells during the telophase. In such cases, two nuclei and two micronuclei form inside the cell during the telophase.
Genotoxicity is assessed with chromosomal aberrations by using a microscope to detect micronuclei formed during the telophase. Fluorescence staining or Giemsa staining is generally used for microscopic observation. As the size of micronuclei is about 1 μm, it is difficult to find them among numerous normally-divided cells under high-magnification observation using a microscope and requires users with specialized skills.
Get detailed information on our products by downloading our catalog.
View Catalog
Typical genotoxicity tests conducted in combination with micronucleus testing
This section introduces other typical genotoxicity tests used to assess genotoxic properties, including DNA-damaging and clastogenic properties. In some cases, to increase the accuracy of assessments, a micronucleus test is combined with another genotoxicity test with a different method of testing and detection characteristics.
Comet assay
A comet assay is an in vivo assay that examines a single cell for the detection of early DNA damage, and is also called a single cell gel electrophoresis assay. In this assay, the isolated cell specimen is electrophoresed in alkaline gel. If there is DNA damage in the nucleus of the specimen cell, a comet-like pattern of DNA breakage and migration can be observed. The greatest difference with in vivo micronucleus assay is the ability to test for DNA damage without cell division of the assessment target organ due to the use of a single cell. Another characteristic is that a comet assay allows for assessment with a variety of organs and tissues, including non-dividing cells. Comet assay has high sensitivity for genotoxic carcinogens and high specificity for non-carcinogenic substances.
A combination of a comet assay and micronucleus test using the same animal is considered to be a rational testing method that achieves a reduction in both the number of animals used for testing and testing time.
Chromosome aberration test
A chromosome aberration test is an in vitro assay that examines chromosomal aberrations by administering a test substance to cultured mammalian cells, such as CHL/IU in the proliferation phase, or to a chromosome specimen of human lymphocytes. The greatest difference from micronucleus tests, which examine the formation of micronuclei in the telophase, is that chromosome aberration tests look for the presence of chromosomal aberrations using chromosome specimens in metaphase. Chromosomal aberrations detected by this test include structural aberrations, such as deletions (breakage in one of the chromatids) and reciprocal translocations (segments from two different chromosomes have been exchanged), and numerical aberrations, such as an extra chromosome or a deficiency in chromosomes.
If no mutagenicity is found as a result of the chromosome aberration test, which checks for chromosomal aberrations in metaphase, a micronucleus test may be conducted to examine the telophase as a secondary screening test.
Ames test (Bacterial reverse mutation test)
The Ames test, also called the bacterial reverse mutation test, is a test intended for the detection of genotoxic carcinogens. Mutation induction is detected when a test substance is given to an autotrophic mutant strain that cannot produce amino acids on its own and the test substance’s mutagenicity allows it to form colonies by “returning to” prototrophic strains that can produce amino acids. In an in vitro assay using Salmonella typhimurium or Escherichia coli, these bacteria are cultured on a soft agar plate that does not contain amino acids. The colonies are counted to determine whether they are positive or negative based on the proportion of plates with colonies.
When testing specifically to identify genotoxic carcinogens, if the Ames test (detecting genetic mutation) result is positive, an in vivo micronucleus test may be used as a secondary screening test.
We’re here to provide you with more details.
Reach out today!

Observing micronuclei with a fluorescence microscope
Generally, when observing micronuclei in a micronucleus test, a microscope is used for visual observation, manual counting, and measurement. However, in order to obtain statistically valid data for a micronucleus test, a significant number of cells must be observed at high magnification in a wide field of view. Picking out and manually counting micronuclei, which are as small as 1 μm, among a vast number of normal divided cells in numerous enlarged fields of view requires a high degree of skill and is also very laborious. It is also a huge problem that it takes a long time to obtain test results.
The solution: Observing micronuclei using a fluorescence microscope
The BZ-X all-in-one fluorescence microscope is the solution to the problems that arise in microscopic observation for genotoxicity tests, including micronucleus tests. The image stitching function, which can automatically capture multiple high-magnification images and assemble them, easily offers high-resolution images over a wide field of view. With just a single click on the navigation image on the monitor, users can easily bring up the areas that they want to see in an enlarged or wide field of view, allowing for quick discovery and observation of micronuclei.
The masked measurement function and detection target size specification function of the Hybrid Cell Count can easily extract and quantify micronuclei, which not only reduces the user’s workload, but also significantly shortens the time required to obtain test results.
The high-sensitivity, cooled CCD monochrome camera used in the BZ-X is optimal for high-definition fluorescence microscopy and can be quickly switched to color observation mode for optimal brightfield observation. This allows a single BZ-X to support a variety of tests, including micronucleus tests, comet assays, other genotoxicity tests, and other general microscopic tests. In addition to rationalizing equipment space in the laboratory, the BZ-X can also be used in bright environments thanks to its built-in darkroom, which allows for flexible installation and use.
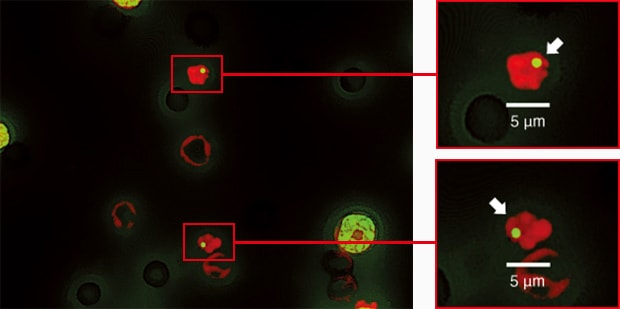
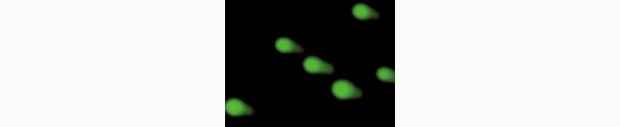
Image stitching and quantification can also be used when performing a comet assay, another genetic toxicology test.
Using the All-in-One Fluorescence Microscope BZ-X
- The image stitching function allows you to automatically capture images with both high resolution and a wide field of view.
- The testing staff only needs to find micronuclei by looking at the captured high-definition, large-field-of-view images on the monitor, which significantly reduces their work load.
- The masked measurement function and detection target size specification function of Hybrid Cell Count can easily extract and quantify micronuclei, significantly reducing the time required to obtain the test results.
- In addition to high-definition fluorescence microscopy, the BZ-X also offers brightfield observation by switching to color observation mode, so it can support a variety of microscopic tests.
- As it does not need a separate darkroom for fluorescence microscopy, it is free of installation location limitations. Additionally, the BZ-X offers a variety of observation approaches in a single device, helping save space by reducing the total equipment footprint.