Fluorescence Microscopes
Cells divide when maintaining, growing, or creating life. Capturing and observing the process of live cell division in chronological order is very important for a variety of research. This section introduces examples of time-lapse imaging that captures the process of live cell division, using actual time-lapse video and images recorded with a fluorescence microscope.
Get detailed information on our products by downloading our catalog.
View Catalog
The purpose of observing the process of cell division
The cells of organisms grow while repeating somatic cell division*1. Meiosis*2 occurs during the formation of germ cells. A lot of research on the cells of living organisms requires observation and evaluation of these division processes in chronological order.
For example, research on diseases and therapeutic drugs (drug discovery screening) observe cell behavior of tissues or organs in the pathological or healing processes. In regenerative medicine, there is active experimentation and research for pluripotent stem cells that can differentiate into any type of cell constituting various tissue and organs, such as iPS cells. In the evaluation and presentation of experiment results in the researches on stem cell differentiation and cell division, the key issue is how to clearly capture the process of chromosome division in live cells.
Meiosis is also observed to identify the causes of diseases such as germ cell hypoplasia and abnormal chromosome partitioning, as well as to understand the molecular mechanism of sexual reproduction and its origin. In gene expression experiments using Messenger RNA (mRNA), which codes the sequence information of proteins copied from DNA, the division process of germ cell nuclei is observed to identify the molecular mechanism of meiosis and the division controlling factors in order to determine the conditions that stabilize mRNA functions.
*1: Somatic cell division is the process in which one mother cell is divided into two daughter cells. An animal cell grows in the cell cycle consisting of the interphase (G1, S, and G2) and the mitotic (M) phase. In the M phase, chromatin partially condenses to become thin chromosomes and the nuclear envelope breaks down. Small asters are formed around the centrosomes (Prophase). Spindle fibers stretch from the two small asters and bond with kinetochores. At the same time, chromatids are formed (Prometaphase). The chromosomes are arranged around the equator (Metaphase). The separated chromatids (daughter chromosomes) move toward the opposite poles. Cytokinesis forms the nuclear envelope and nucleolus in these chromatids, making two daughter cells (Telophase). Each cell enters the interphase (G1 phase).
*2: Meiosis is a type of cell division that occurs when germ cells (e.g. sperm, eggs) are formed. The cell nuclei are divided twice in mitosis, which decreases the number of chromosomes by half. This division is also called reduction division and, in animal germ cells, maturation division. In the first division, homologous chromosomes pair up with each other and then are separated. In this process, the number of chromosomes decreases by half. In the second division, the cells are simply divided, forming four cells with half the number of chromosomes. Germ cells will obtain the normal number of chromosomes when undergoing fertilization.
We’re here to provide you with more details.
Reach out today!

Examples of time-lapse imaging of cell division
In typical observation of the process of cell division, time-lapse imaging that captures fluorescence images of live cells at specified time intervals is used. To perform experiments and imaging successfully, it is important to minimize damage to the live cells under fluorescent observation.
The following sections introduce important points to consider in imaging and the advantages of using the BZ-X, using time-lapse video and images of cell division recorded with KEYENCE’s All-in-one Fluorescence Microscope BZ-X.
Time-lapse video of cell division
To reduce live cell damage that commonly occurs in time-lapse imaging of fluorescent observation, the BZ-X automatically controls the excitation light shutter while not recording.
In combination with its high-resolution cooled CCD monochrome camera that can capture clear images with less noise across short and long wavelengths, the BZ-X can capture faint fluorescent signals over long periods while minimizing the duration of irradiation by excitation light.
Another issue in time-lapse imaging is that the cells move outside the field of view while chromosome division is being observed at high magnification. The BZ-X can adjust the imaging position in the X-, Y-, and Z-axis directions even during recording. This adjustment uses previously taken images, preventing photobleaching and decreased cell activity from excitation light irradiation.
Additionally, with the user-friendly operation screen interface, even fluorescence microscope beginners can easily configure operation and time-lapse imaging settings to perform time-lapse recordings.
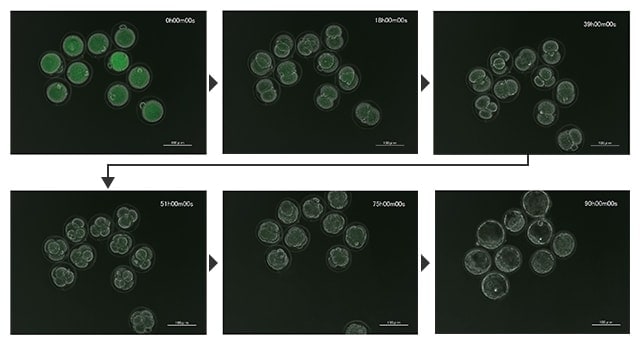
Time-lapse images of development of an early embryo in a mouse egg
Time-lapse images of development of an early embryo in a mouse egg (phase contrast + fluorescence at low magnification)
Imaging conditions: Phase contrast 20x, overlay (phase contrast + GFP), 120-hour recording in 3-hour intervals
The BZ-X can perform high-definition time-lapse imaging while reducing damage to live cells. Photobleaching can also be reduced, which allows for the recording of cells as they are without losing activity. In time-lapse imaging of multiple cells in a wide field of view at low magnification, it is also possible to clearly record the division process of each cell.
The BZ-X, which supports fluorescence, brightfield, and phase contrast imaging with a single unit, is equipped with a real-time overlay function that superimposes a phase contrast image on a fluorescence image on a single screen in real time, as shown in the images above. Imaging conditions, such as the observation method and exposure time, can be saved in different channels, which saves the time and effort required for imaging adjustment and reshooting.
To perform time-lapse imaging successfully, it is necessary to determine, in advance, the conditions with which to image targets and signals clearly throughout the recording. This preparation needs to be done effectively in a short time because prolonged condition setting may lead to photobleaching in experiments.
Using the All-in-One Fluorescence Microscope BZ-X
- The high-sensitivity optical system can capture even faint fluorescent signals at high resolution with less noise. Additionally, the low photobleach mode automatically stops excitation light irradiation when it isn’t needed. Long duration time-lapse imaging is possible with reduced damage to live cells.
- Position adjustment in the X-, Y-, and Z-axis directions during time-lapse capture is possible. This adjustment uses previously taken images, eliminating concerns about photobleaching and decreased cell activity.
- The system supports fluorescence, brightfield, and phase contrast imaging with a single unit. The optimal settings can be configured quickly and effectively with the real-time overlay function that allows for imaging checking with superimposed images captured under different observation and recording conditions saved in different channels.
- With a user-friendly interface and electronic control that requires only a mouse, even beginners can easily perform fluorescence observation and time-lapse recording.