Industrial Laser Marking Systems / Laser Markers
8 Types of Common Lasers
-
Tags:
- Automotive , Medical , Semiconductor
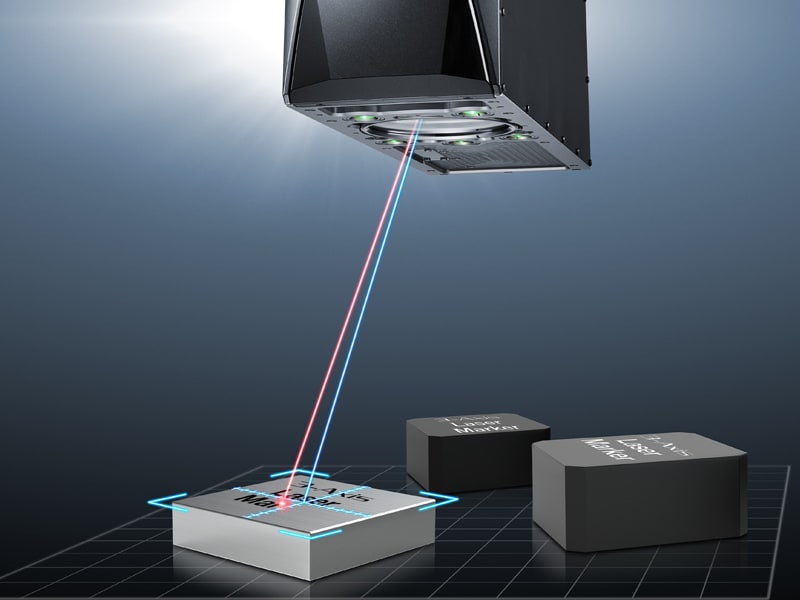
Dating back to 1960, when Theodore Maiman invented the first laser, this form of light has evolved into a tool used across industries. From medical treatment to manufacturing processing, the power of lasers is used across a myriad of industries and for many types of applications.
When most think of lasers, the ubiquitous red light lasers often come to mind. However, there are many different types of lasers. In fact, there are over eight types of lasers across three categories! In this blog, we'll explore each category of laser, what the category entails, uses for lasers, and eight different types of laser machines that make up these categories.
Classification of Industrial Lasers
Based on their gain medium, industrial lasers can be divided into three primary groups: liquid, gas, and solid-state systems. Every category has special benefits for particular production procedures. Depending on the medium, laser marking methods range greatly among these categories in terms of beam quality and power output.
The choice between types of laser machines depends on material properties, precision requirements, and production volume. Manufacturers must consider wavelength compatibility with their target materials when selecting equipment.
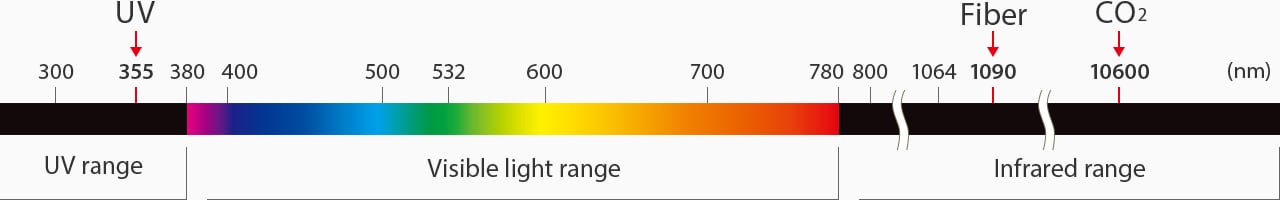
Light wavelength distribution map
We’re here to provide you with more details.
Reach out today!

Solid-State Lasers
Solid-state lasers are types of lasers that use a solid material as its gain medium. The most common solid-state laser media are yttrium aluminum garnet (YAG) and yttrium vanadate (YVO4). When a laser diode shines light on the doped material, the neodymium atoms are excited and emit light. This light is then amplified by the material, focused and emitted as a laser beam.
YVO4 requires large amounts of energy to produce a stable high-output energy amount. This medium has recently taken over in popularity compared to YAG mediums. It's known for assisting in the high-quality marking of metals, plastic, and ceramic.
YAG mediums require less energy than YVO4 lasers, which is why they reigned popular as the universal marking structure for so long. However, because of their need for less energy, this structure is not as stable as YVO4. YAG is known for more brute force and faster marking.
Fiber lasers dominate modern production environments due to their exceptional reliability and minimal maintenance needs. These systems deliver consistent beam quality over thousands of operating hours. Their compact form allows easy integration into existing production lines without major facility modifications.
Within the solid-state laser category are three different types of lasers: Green, UV, and Fiber.
Green Laser
Green lasers, also known as second-harmonic lasers, are notable for their specialized ability to process at a micron level. The laser begins at a 1064 nm wavelength and then passes through a nonlinear crystal for conversion into a 532 nm wavelength. With the much shorter wavelength that appears green to the human eye, intricacy is a green laser's special power. Green lasers are used as pointing devices and for micron-level marking and processing.
UV Laser
UV lasers, also known as third-harmonic lasers, are a specialized type of solid-state laser that uses “cold marking.” Cold marking refers to a UV laser's strength of absorption that produces very little heat when marking. These lasers start at a 1064 nm wavelength and then pass through two nonlinear crystals to transition to a 355 nm wavelength.
Because of the extremely small beam, small processing and minimal damage are two key components of a UV laser. UV lasers are powerful enough to create high contrast marks on highly reflective materials like copper, gold, and silver.
Fiber Laser
Solid-state fiber lasers are highly regarded for their high average power, fast speed, and excellent cooling efficiency. With the ability to mark on metal, plastic, and ceramic with a 1030-2100 nm wavelength, it's even more impressive that fiber lasers can engrave, anneal, mark, etch, cut, and remove burrs. Fiber lasers have the smallest marking unit size, making them the easiest to retrofit, or integrate in production settings with limited space.
Gas Lasers
Gas lasers are used for machining and marking applications like marking labels, etching plastic and resins, processing, and cutting. These laser types use gas as the medium instead of a solid or liquid. The four types of gas lasers are CO2, He-Ne, excimer, and argon.
CO2 Laser
CO2 gas lasers have the longest wavelength compared to fiber, YAG, and YVO4. CO2 lasers have a wavelength of 10600 nm. Because of the long wavelength, there is more heat transfer than other lasers. Moreover, CO2 lasers are commonly used for cutting materials.
CO2 lasers excel at cutting plastic materials and processing organic compounds. Their longer wavelength generates more heat, which penetrates materials differently than shorter wavelengths. These systems require proper ventilation and gas supply management for optimal performance.
He-Ne Laser
He-Ne gas lasers have a wavelength of 630 nm and appear red to the human eye. He-Ne lasers are commonly known for their use as pointing devices in classrooms or during presentations. These laser types are also used in store barcode scanners and measurement devices. Unlike many other different types of lasers, He-Ne lasers do not do manufacturing processing.
He-Ne systems offer affordability for basic applications but lack the power needed for heavy manufacturing. Their visible red beam makes alignment and setup straightforward for operators.
Excimer Laser
Excimer gas lasers use inert gas and hydrogen gas to produce a short UV wavelength of 193 nm. These lasers are commonly used for dermatology and optometry treatments. In fact, excimer lasers are proven to treat skin conditions like alopecia, psoriasis, leukoderma, and more. These lasers are also used in optometry to vaporize the lens of human eyes.
Argon Laser
Similar to excimer lasers, argon gas lasers are also used for various health conditions, scientific applications, and biomedical research. Argon lasers use a 488 to 514 nm wavelength, and one popular type of argon laser treatment is for glaucoma or diabetic eye disease treatment.
Liquid Lasers
Dye Laser
Dye lasers are liquid lasers with a wavelength of 330 to 1300 nm commonly used for scientific applications. These lasers use organic liquid dyes to power their wavelengths and produce fluorescent light.
Discover more about this product.
Click here to book your demo.
UV Lasers: When and Why to Use Them
When it comes to fragile materials that are unable to withstand heat damage, UV lasers offer unrivaled precision. When marking, its high absorption capabilities keeps materials from warping or discoloring. UV systems are used by electronics makers to identify components without sacrificing circuit integrity.
These systems work best on plastics, glass, and sensitive substrates where thermal effects must be minimized. Types of lasers in the UV category require more careful handling due to their invisible beam properties.
Choosing the Right Laser for Your Application
When choosing an industrial laser, compatibility of materials, manufacturing speed requirements, and quality standards must all be carefully considered. Every kind of laser has unique benefits for particular uses. Automotive manufacturers often prefer fiber systems for their durability and speed.
UV systems are commonly used by medical equipment manufacturers for biocompatible branding that poses no risk of contamination. The choice has a major effect on both the initial outlay and ongoing operating expenses.
Wavelength, Power & Budget Factors
Wavelength determines material absorption characteristics and achievable mark quality. Shorter wavelengths generally provide finer detail but may require higher initial investment. Power requirements scale with production volume and material thickness.
Budget considerations must include consumables, maintenance schedules, and energy consumption over the equipment’s lifetime. Laser marking technologies continue advancing, making older systems less cost-effective over time.
3-Axis CO2 Laser Marker ML-Z9500

World’s first 3-Axis control laser marker
3-Axis Fiber Laser Marker MD-F3000

World’s smallest fiber laser marking unit
Telecentric Laser Marker MD-T1000

World’s first green laser marker with a telecentric lens
3-Axis UV Laser Marker MD-U2000

World’s first UV laser marker with 3-Axis control
3-Axis Hybrid Laser Marker MD-X2000/X2500

World's first laser marker with full-field auto-focus
Contact us to learn more about how our advanced technology can help take your business to the next level.
Contact Us
FAQs
How Does Laser Wavelength Affect the Quality of Marking?
Shorter wavelengths (e.g., UV) produce high-resolution marks on delicate materials. Longer wavelengths like CO2 provide deeper penetration for cutting applications.
Are Certain Lasers Better Suited for Automated Production?
Fiber lasers are often preferred due to low maintenance and integration flexibility. Their solid-state design eliminates gas handling requirements found in CO2 systems.
Can One Type of Laser Handle Both Marking and Cutting?
Some high-power fiber lasers can handle both, but quality depends on application specifics. Dedicated systems typically provide better results for each process.
What Industries Benefit Most From Fiber Lasers?
Automotive, aerospace, and electronics industries rely heavily on fiber lasers. Their precision and reliability meet strict quality standards required in these sectors.
We’re here to provide you with more details.
Reach out today!







